[Image above] Asahi Kasei’s proof-of-concept tests for its acetonitrile-based electrolyte used in lithium-iron phosphate batteries. Credit: Asahi Kasei
By Laurel Sheppard
From portable electronics to electrified transportation, lithium-ion batteries serve as the predominant commercial form of rechargeable battery. Yet as these batteries are adopted into more and more applications, some of their performance limitations are becoming more noticeable, such as their sensitivity to temperature.
In general, fast charging of Li-ion batteries can only take place between 5°C to 45°C (41 to 113°F). Below 0°C (32°F), plating of metallic lithium occurs on the anode that leads to a permanent degradation in performance and safety. Above 50°C (122°F), charging and discharging is subject to gas generation that might cause a cylindrical cell to vent and a pouch cell to swell.
The electrolyte, which is used to transport the lithium ions between the battery’s electrodes, is the main cause of these temperature limitations. The most commonly used electrolyte is made from lithium salt, specifically lithium hexafluorophosphate (LiPF6), in an organic solution, usually some type of carbonate. This electrolyte undergoes changes in physical properties, such as viscosity, at high and low temperatures, which affects the battery’s ability to successfully charge and discharge.
Developing new electrolyte formulas with higher ionic conductivities could help expand the temperature range in which Li-ion batteries can viably operate. One promising material is acetonitrile, an organic compound that is commonly used as a solvent for the production of pharmaceuticals and photographic film. In addition to high ionic conductivity, acetonitrile solutions demonstrate high chemical and oxidative stability.
However, acetonitrile tends to decompose in the presence of graphite, another major battery material used as the negative electrode. So, a challenge for researchers is to find a way to stabilize acetonitrile in the presence of graphite.
One scientist aiming to overcome this challenge is Akira Yoshino, fellow of Asahi Kasei Corporation and professor at Meijo University, Japan. Yoshino is one of three luminaries who received the 2019 Nobel Prize in Chemistry for the development of Li-ion batteries. He built on the discoveries of M. Stanley Whittingham of Binghamton University and John B. Goodenough of the University of Texas at Austin to develop the basic configuration of Li-ion batteries, which were commercialized in 1991 by Sony and then by Asahi Kasei and Toshiba in 1992.
In 2021, Yoshino and colleagues at Asahi Kasei published an open-access paper describing their solution to suppress the reductive decomposition of acetonitrile.
First, they prepared the nonaqueous, acetonitrile-based electrolytes by dissolving various lithium salts in acetonitrile. The ionic conductivity showed a maximum (>15 mS/cm) at a 1.3 mol concentration of the salts in 1 liter of solvent. Then, they added a combination of vinylene carbonate and ethylene sulfite to the electrolyte, which formed a solid electrolyte interface between the anode and electrolyte and effectively suppressed the decomposition reaction.
Degradation during cycling increased once acetonitrile content became greater than 35 vol.%. Conversely, the cycling performance was similar to a reference carbonate-based electrolyte when acetonitrile content was reduced to 10 vol.%. The cycling performance was improved for all acetonitrile concentrations by adding pyridine as a stabilizer.
In the original study, all electrolytes showed a superior performance at −40°C, retaining a capacity between 37 to 54 milliampere-hours per gram mass at 1°C. In contrast, the carbonate-based reference electrolyte showed no remaining capacity at 1°C.
Other potential benefits of the acetonitrile-based electrolyte include
- Enables the usage of thicker electrodes, thus increasing capacity.
- Reduces battery pack size while maintaining power output, contributing to higher battery energy density and lower overall pack cost.
- Reduces the time it takes to charge a battery from 10 to 80% of its full charge by up to 50%, depending on the cell design.
- Manufacturing process is similar to current state-of-the-art liquid electrolytes, making wide adoption possible.
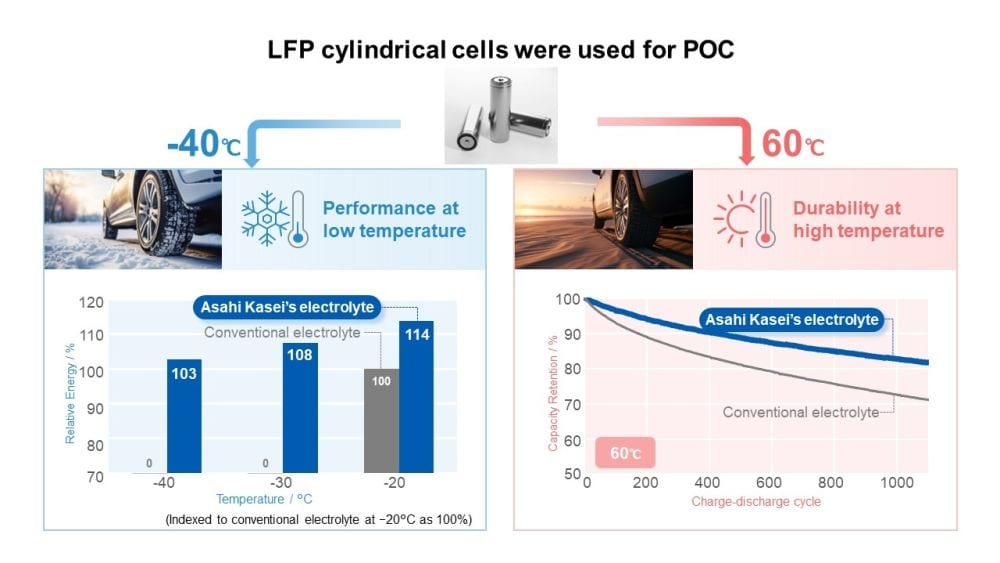
In June 2024, Asahi Kasei announced that its researchers had achieved proof-of-concept with this proprietary acetonitrile-based electrolyte, in collaboration with a battery maker, using cylindrical lithium-iron phosphate (LFP) batteries. The tested cylindrical cells showed high power at −40°C and double the cycle life compared to typical Li-ion batteries at 60°C (140°F), which is “a technological breakthrough,” according to Kazuya Noda, senior general manager of Asahi Kasei’s Innovation Strategy Center, in the press release.
Commercialization of the acetonitrile-based electrolyte technology is targeted for 2025. In the press release, Noda says “By licensing the electrolyte technology to [Li-ion battery] manufacturers worldwide, Asahi Kasei aims to contribute to lower cost and more compact battery systems, which are a key driver to achieve a more sustainable society.”
